Project Description
J Cell Sci. 2014 Jan 1;127(Pt 1):182-90. doi: 10.1242/jcs.134718. Epub 2013 Nov 4.
Proneural proteins Achaete and Scute associate with nuclear actin to promote formation of external sensory organs.

Author information
Yun-Ling Hsiao1,2, Yu-Ju Chen1,2, Yi-Jie Chang1,2, Hsiao-Fong Yeh2, Yi-Chun Huang3, Haiwei Pi1,2
- Graduate Institute of Biomedical Sciences, College of Medicine, Chang Gung University, 259 Wen-Hwa 1st Road, Kwei-Shan, Tao-Yuan 333, Taiwan
- Department of Biomedical Sciences, College of Medicine, Chang Gung University, 259 Wen-Hwa 1st Road, Kwei-Shan, Tao-Yuan 333, Taiwan
- Graduate Institute of Life Sciences, National Defense Medical Center, Taipei 114, Taiwan
論文介紹
 在果蠅外感官器官的發育過程中,其中最重要的步驟是從前驅神經細胞群中選出一顆感覺器官母細胞並賦予其神經的命運。原神經基因 achaete (ac) 和 scute (sc) 轉譯出鹼螺旋-轉-螺旋型轉錄蛋白,對於此步驟為最主要的控制者。對於了解原神經蛋白 Ac 和 Sc 開始並執行外感官器官的形成是透過什麼樣的分子機制,如何透過結合不同的輔助因子進而改變其功能和特性是很重要的問題。
在果蠅外感官器官的發育過程中,其中最重要的步驟是從前驅神經細胞群中選出一顆感覺器官母細胞並賦予其神經的命運。原神經基因 achaete (ac) 和 scute (sc) 轉譯出鹼螺旋-轉-螺旋型轉錄蛋白,對於此步驟為最主要的控制者。對於了解原神經蛋白 Ac 和 Sc 開始並執行外感官器官的形成是透過什麼樣的分子機制,如何透過結合不同的輔助因子進而改變其功能和特性是很重要的問題。
鹼螺旋-轉-螺旋型原神經蛋白透過轉錄調控機制來促進神經發育。對於原神經蛋白如何調控組織專一性的神經性前驅基因的表現已了解很多,但是藉由什麼樣的轉錄機制去活化下游基因的表現卻不清楚。
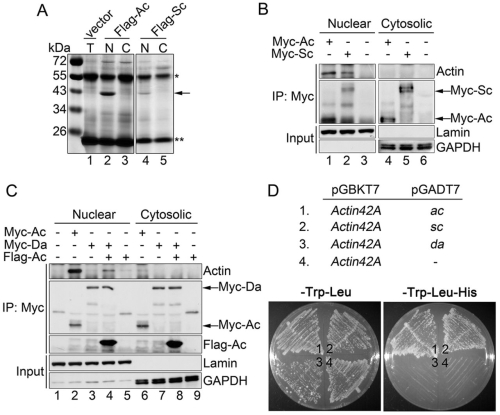
果蠅的原神經蛋白 Achaete (Ac) 和 Scute (Sc) 是藉由活化神經性前驅基因的表現來誘發外感官器官母細胞的形成。利用免疫共沈澱和質譜分析的方式,我們發現在果蠅的 S2 細胞中,核內的肌動蛋白會與 Ac 和 Sc 結合。而 Daughterless (Da) 需與原神經蛋白 Ac 和 Sc 鍵結成異源二聚體才能和核內的肌動蛋白結合。利用酵母菌雙雜交實驗也發現 Ac 和 Sc 與肌動蛋白的專一性結合並不需要果蠅其他的因子參與。
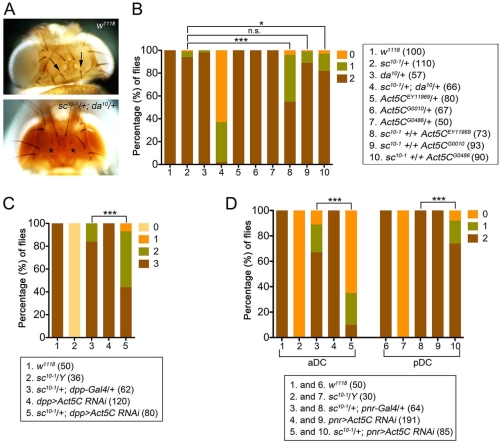
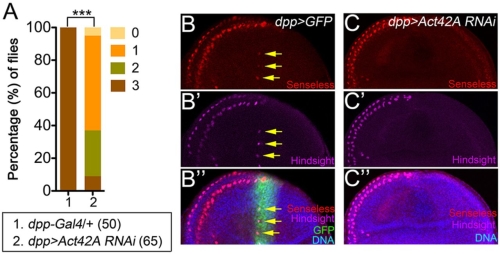
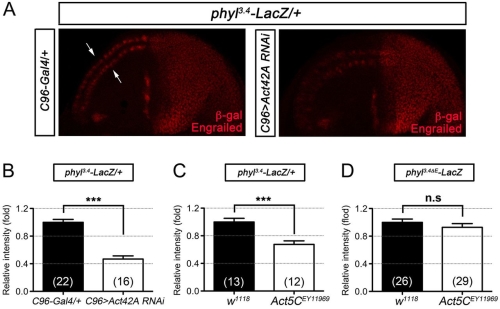
接下來,我們想知道肌動蛋白對於外感官器官的形成是否重要。在減少肌動蛋白基因的活性下,神經性前驅基因的表現及神經前驅細胞的形成皆會受損。此外,藉由額外表達會進核的肌動蛋白迫使核內肌動蛋白的量增加,進而受 Ac/Da 調控的基因轉錄活性會隨著核內肌動蛋白量的增加而隨之升高,同時並促進外感覺器官的形成。
在這篇文章中,我們從活體內及試管中的觀察,發現核內的肌動蛋白,對於增加原神經蛋白的轉錄活性進而促使神經前驅細胞的分化,扮演一個重要的關鍵角色。
Abstract
Basic helix-loop-helix (bHLH) proneural proteins promote neurogenesis through transcriptional regulation. Although much is known about the tissue-specific regulation of proneural gene expression, how proneural proteins interact with transcriptional machinery to activate downstream target genes is less clear. Drosophila proneural proteins Achaete (Ac) and Scute (Sc) induce external sensory organ formation by activating neural precursor gene expression. Through co-immunoprecipitation and mass spectrometric analyses, we found that nuclear but not cytoplasmic actin associated with the Ac and Sc proteins in Drosophila S2 cells. Daughterless (Da), the common heterodimeric partner of Drosophila bHLH proteins, was observed to associate with nuclear actin through proneural proteins. A yeast two-hybrid assay revealed that the binding specificity between actin and Ac or Sc was conserved in yeast nuclei without the presence of additional Drosophila factors. We further show that actin is required in external sensory organ formation. Reduction in actin gene activity impaired proneural-protein-dependent expression of the neural precursor genes, as well as formation of neural precursors. Furthermore, increased nuclear actin levels, obtained by expression of nucleus-localized actin, elevated Ac–Da-dependent gene transcription as well as Ac-mediated external sensory organ formation. Taken together, our in vivo and in vitro observations suggest a novel link for actin in proneural-protein-mediated transcriptional activation and neural precursor differentiation.