Project Description
PLoS Genet. 2014 Nov 13;10(11):e1004760. doi: 10.1371/journal.pgen.1004760. eCollection 2014 Nov.
The COP9 signalosome converts temporal hormone signaling to spatial restriction on neural competence.

Author information
Yi-Chun Huang1, Yu-Nung Lu2, June-Tai Wu3, Cheng-Ting Chien4, Haiwei Pi2.
- Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan; Department of Biomedical Sciences, College of Medicine, Chang Gung University, Tao-Yuan, Taiwan; Insitute of Molecular Biology, Academia Sinica, Taipei, Taiwan.
- Department of Biomedical Sciences, College of Medicine, Chang Gung University, Tao-Yuan, Taiwan.
- Institute of Molecular Biology, National Taiwan University, Taipei, Taiwan.
- Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan; Insitute of Molecular Biology, Academia Sinica, Taipei, Taiwan.
論文介紹
動物用各式各樣的感覺器官來感受外界環境。這些感覺器官必須長在正確的位置來執行正常功能。在這篇研究裡面,我們用果蠅外感覺器官作為模式來研究感覺器官生長位置的決定機制。有趣的是我們發現感覺器官位置的決定居然是靠著控制蛻皮激素活性改變的時間點。這種發育過程中時間的變化影響空間分布的機制是我們這篇研究裡面一個非常重要的發現。
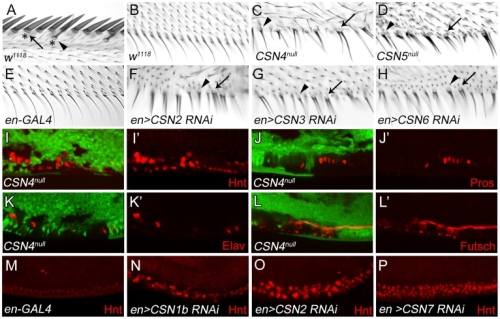
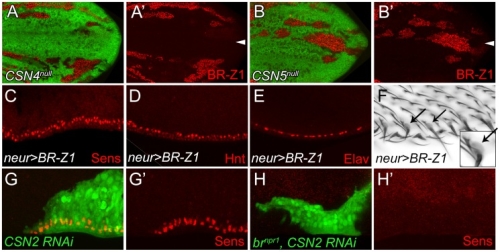
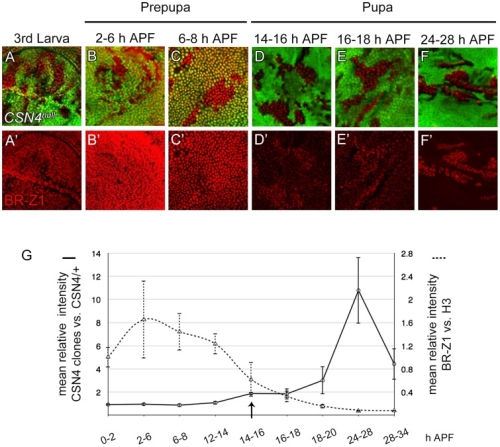
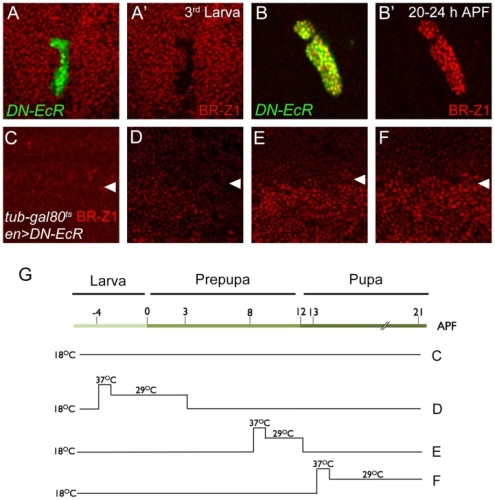
在正常的果蠅翅膀邊緣,外感覺器官只分布在前緣。這種外感覺器官主要是用來感受機械性刺激,比如觸摸或空氣流動。後緣只有長出細毛狀的構造。但是我們發現在 COP9 複合體的突變中,後緣的細毛會變成外感覺器官。這是因為 COP 複合體會抑制蛻皮激素下游基因 BrZ1 的表達,進而抑制神經母細胞在後緣的形成。有趣的是,COP9 複合體的抑制作用只發生在特定的時間點。這樣的時間控制讓COP9複合體剛好可促進後缘感覺器官母細胞的退化,但是不影響前緣母細胞的形成。且這個時間點剛好也是蛻皮激素接受器從轉錄活化子變成抑制子的關鍵時間。而蛻皮激素接受器需要和 COP9 複合體結合才能變成轉錄抑制子。最後我們又証明 COP9 複合體的抑制作用是由於其促進 deneddylation 的作用有關。
因此我們的研究證明 COP9 複合體藉由改變蛻皮激素接受器的轉錄特性來影響神經母細胞在空間上面的分佈。我們這篇研究在發表時,同時也被選出刊登在 PLOS Genetics 的首頁。
Abstract
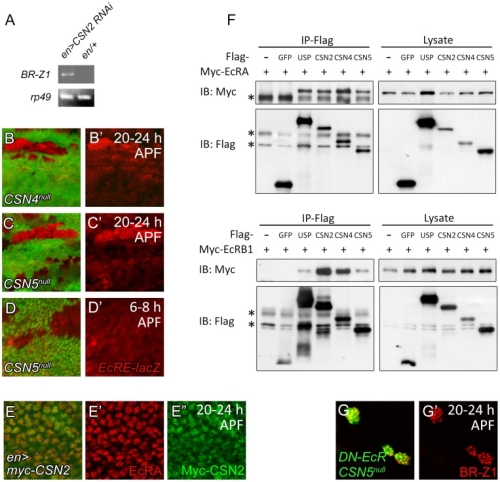
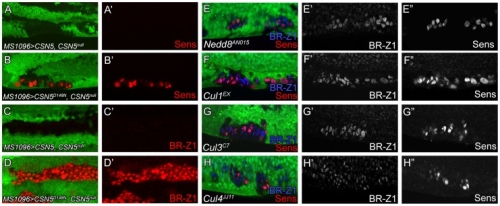
During development, neural competence is conferred and maintained by integrating spatial and temporal regulations. The Drosophila sensory bristles that detect mechanical and chemical stimulations are arranged in stereotypical positions. The anterior wing margin (AWM) is arrayed with neuron-innervated sensory bristles, while posterior wing margin (PWM) bristles are non-innervated. We found that the COP9 signalosome (CSN) suppresses the neural competence of non-innervated bristles at the PWM. In CSN mutants, PWM bristles are transformed into neuron-innervated, which is attributed to sustained expression of the neural-determining factor Senseless (Sens). The CSN suppresses Sens through repression of the ecdysone signaling target gene broad (br) that encodes the BR-Z1 transcription factor to activate sens expression. Strikingly, CSN suppression of BR-Z1 is initiated at the prepupa-to-pupa transition, leading to Sens downregulation, and termination of the neural competence of PWM bristles. The role of ecdysone signaling to repress br after the prepupa-to-pupa transition is distinct from its conventional role in activation, and requires CSN deneddylating activity and multiple cullins, the major substrates of deneddylation. Several CSN subunits physically associate with ecdysone receptors to represses br at the transcriptional level. We propose a model in which nuclear hormone receptors cooperate with the deneddylation machinery to temporally shutdown downstream target gene expression, conferring a spatial restriction on neural competence at the PWM.M.